Research
Foldamer design
Molecular Recognition within foldamers
Biological applications of foldamers
Helical aromatic oligoamide foldamers have been developed in our lab with fully cationic side chains conferring them with water solubility and and cell penetration properties.[1] Advances in synthetic folded architecture design together with the finding that some aromatic backbones also fold in water have opened avenues toward selective foldamer–biomolecule interactions and foldamers that interfere with biological functions.
Development of new synthetic tools: Solid Phase Synthesis (SPS) of aromatic oligoamide foldamers
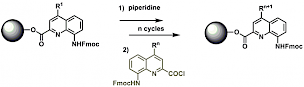
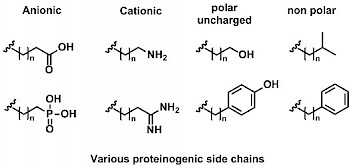
Solid Phase Synthesis which is widely used to provide synthetic foldamers such as β-peptides or peptoids has recently been developed in our lab in order to obtain helical aromatic amide foldamers.[2] The optimization of biological activities often proceeds through the parallel synthesis of multiple variants possessing different proteinogenic side chains, which is best achieved on solid phase. Typically, monomers bearing proteinogenic side chains are prepared as Fmoc-protected amino acids, activated as acid chlorides and coupled on resin using microwave irradiation to enhance reaction rates.
 |
 |
Foldamers/Biomolecules interactions
Synthetic foldamers, due to their medium size (typically in the 0.5-5 kDa range) and well-defined structure in solution, appear as potent candidates to serve as scaffolds bearing proteinogenic side chains that would recognize biomacromolecules. In this respect aromatic oligoamides constitute particularly attractive candidates and alternatives to peptidic and oligonucleotidic backbones as their conformations are highly stable in water and reliably predictable. In addition, helical cationic aromatic oligoamides show high resistance towards enzyme degradation and cell penetration properties.[1]
Foldamers/DNA interactions
Cationic aromatic structures are potent candidates to interact with nucleic acids in water. We have discovered that helical oligoamides strongly and selectively interact with the loops or grooves of G-quadruplex DNA, unlike conventional ligands which normally stack on top of G-tetrads.[3] Directed DNA evolution (SELEX) against a multiturn helical aromatic amide foldamer having cationic side chains exclusively yielded G-quadruplex aptamers, confirming the selectivity of this interactions.[4]